Revolutionising Clinical Trials Through Active Tracking
Clinical trials stand at the heart of pharmaceutical research and development shaping the future of medicine. When trials were being conducted and monitored in the past using traditional methods, many challenges could arise, and there was a massive scope for improvement as it didn't achieve the desired outcomes. Now, this is not the case. Imagine a world where these trials become more efficient, safer for patients, and fully compliant with regulations. It's no longer a distant dream but a reality that active tracking brings to life.
Today, we will explore a transformative approach that is rewriting the rules of clinical trials – active tracking. We will discuss the significance of this technology and the impact on clinical trials and its role in promoting patient safety and regulatory compliance.
The Current Landscape Of Clinical Trials
Traditional clinical trials have long been the backbone of pharmaceutical advancement. However, it previously relied on painstakingly manual processes and the industry had grappled with challenges that hindered its potential. The inefficiencies, patient safety concerns, and regulatory tightrope that trials navigate have spurred a need for change.
The need for transformation is now, as the time has come, to reimagine the way we approach clinical trials.
The Role Of Active Tracking In Clinical Trials
Enter the concept of active tracking – a game-changer in clinical trial management. Imagine a real-time view into every step of the pharmaceutical supply chain, from manufacturing to distribution. It is precisely what active tracking offers. It all happens by employing technologies like GPS and RFID. Active tracking ensures seamless traceability of pharmaceutical products. Not only does it prevent loss, but it enhances patient safety through accurate monitoring and temperature control.
Picture this: a critical drug is transported under controlled conditions and its temperature is monitored every step of the way. It isn't science fiction – it's the future we're stepping into. If you want to make this scenario a reality for your business, you can employ our temperature data logger solutions. You will be able to monitor in real-time the condition of your package at each stage of the supply chain. Additionally, it will equip you with the power of instant alerts if any breaches occur that disturbs the controlled conditions of your package allowing to take necessary action at that time.
Empowering Efficiency Through Active Tracking
As clinical trials navigate the maze of protocols and procedures, delays are more than inconveniences - they're costly setbacks. Real-time monitoring emerges guiding trials to efficient outcomes through the data that is collected. Another facet of this revolution that targets the heart of clinical trial inefficiencies.
Imagine a trial where data flows seamlessly, offering instant insights. Delays due to manual data collection and analysis become a thing of the past. Instead, data points are streamed in real-time, enabling swift adjustments and maximising efficiency. Such optimisation minimises delays, accelerates the trial process, and ultimately brings us closer to medical breakthroughs.
Ensuring Regulatory Compliance & Patient Safety
Regulatory compliance and patient safety are non-negotiable elements in clinical trials. It also ensures that clinical trials are conducted ethically and transparently in pharmaceutical research. With patient safety at the forefront, active tracking serves as allies in maintaining this compliance. By offering real-time insights and data, these technologies provide auditors and regulators with an accurate depiction of trial conditions and processes.
Consider a regulatory inspection where historical data is no longer the only source of information. With real-time monitoring, trial conditions lay bare, leaving no room for ambiguity. Moreover, patient safety is enhanced by meticulously monitoring sensitive
pharmaceutical products, ensuring their efficacy.
IoT & Temperature Data Loggers' Role In Active Tracking
The Internet of Things (IoT) and temperature data loggers lie at the heart of these transformations in clinical trials. IoT serves as the driving force behind real-time monitoring, enabling seamless data collection, transmission, and analysis. Temperature data loggers, on the other hand, emerge as the unsung heroes as they ensure that temperature-sensitive pharmaceuticals are kept within the required range throughout their journey.
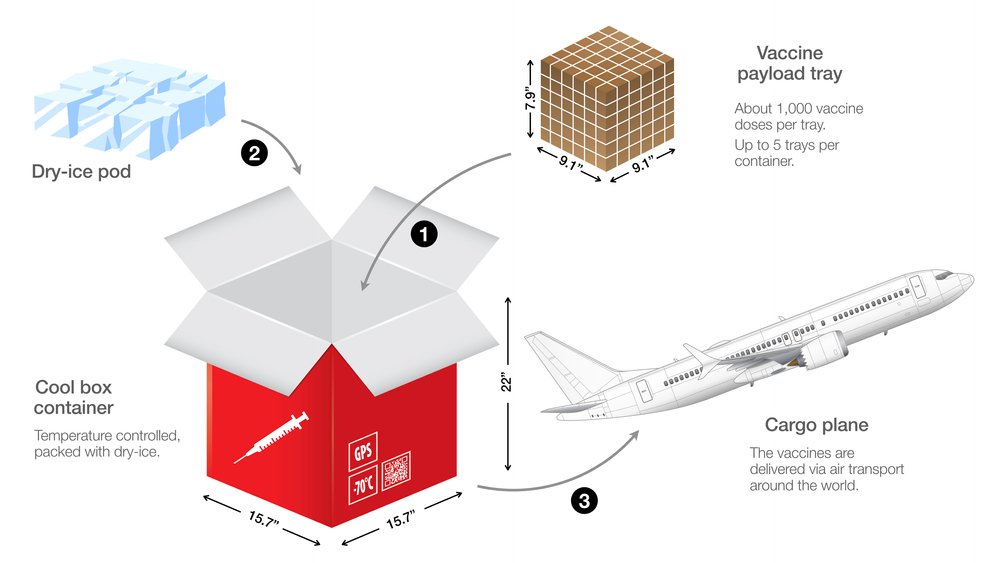
Picture this: IoT-enabled temperature data loggers embedded within shipments of vaccines. These devices continuously track temperature fluctuations immediately alerting stakeholders if conditions deviate from the optimal range. This integration ensures that vaccines maintain their efficacy, ultimately saving lives.
Read more about - The Impact of IoT Enabled Supply Chain Visibility Solutions.
Revolutionising Clinical Trials For A Better Future
In the grand symphony of clinical trials, active tracking, regulatory compliance, patient safety, and IoT converge to compose a harmonious melody of progress. IoT technology and real-time data once a distant dream, now stand as tangible tools that revolutionise the landscape of clinical trials.
As you've journeyed through the transformative landscape of clinical trials, a question naturally arises - how can your team embrace this future? The answer is simple: Adapt Ideations' temperature data logger solutions. These solutions stand as the bridge between today's trials and tomorrow's triumphs.
If you're ready to embark on this transformative journey, take the first step and book a discovery call today. Join us in shaping a future where clinical trials are efficient, safe, and poised to deliver medical marvels. In this era of transformation, innovation isn't just an option - it's a necessity.
Discover the Future of Clinical Trials with Adapt Ideations.
FAQs about data loggers for pharmaceuticals
Get in touch with us today at enquiries@adaptideations.com for further information on our solutions.
Share Our Post.
Awards & Recognition

Best Temperature Monitoring Solution Provider
Awarded by India Biologics & Vaccines Outstanding Industry Awards 2022

Adapt Ideations Recognised As A Supply Chain Leader
by Alcott Global on Supplify's Supply Chain Tech Map 2.0

Related Articles.