How Connected Devices Are Revolutionising The Pharmaceutical Industry
In recent years, the
pharmaceutical and healthcare industries have seen innovation driven by connected devices. Connected devices or the internet of things (IoT) is a network of interconnected devices with sensors that collects and transmits data. Connected devices have the potential to improve patient outcomes in the pharmaceutical industry, boost efficiency, reduce costs, enhance data collection, and improve drug development.
What Are Connected Devices?
Smartphones, computers, and other IoT devices are examples of connected devices which communicates with other devices and networks. These gadgets employ sensors to gather data and transmit it to other gadgets or networks. The pharmaceutical sector is changing due to connected devices, which enables pharmaceutical companies, physicians, and patients to more effectively monitor and manage medical problems and products.
Connected Devices & The Pharmaceutical Industry
Connected devices have transformed the medical sector by providing real-time data to all stakeholders throughout pharma supply chains all the way to end users, allowing them to make decisions backed by data. The pharmaceutical industry is also using this technology to enhance the process of developing new drugs and patient outcomes.
Pharmaceutical companies frequently use connected devices, such as
temperature data logger solutions. These tools are used to keep track of temperature-sensitive goods throughout storage and transportation to make sure they stay within a certain temperature range. It is essential to guarantee that pharmaceutical products' efficacy remains high and are safe to use.
How Connected Devices Are Changing The Pharmaceutical Industry?
The pharmaceutical industry is one of the world's largest and most strictly regulated industries. It has reached a global valuation of $1.48 trillion in 2022. Still, it has to follow laws and regulations covering everything from pharmaceutical development to clinical trials to the marketing of products. The industry is also dealing with a number of difficulties, such as rising costs, escalating competition, and shifting patient needs.
Thanks to connected devices, many of these challenges can be solved. Here are some steps on how IoT is driving change in the pharmaceutical sector.
1. Real-Time Data Collection
Real-time data collection, which is essential for drug development, is made possible by connected devices. Pharmaceutical companies can gather information on the efficacy and safety of their products, allowing them to make decisions backed by data. Additionally, this information can be used to spot any possible drug safety issues, further improving patient outcomes. One such example is the use of temperature monitoring solutions to track in real-time the conditions of life saving vaccines when being transported.
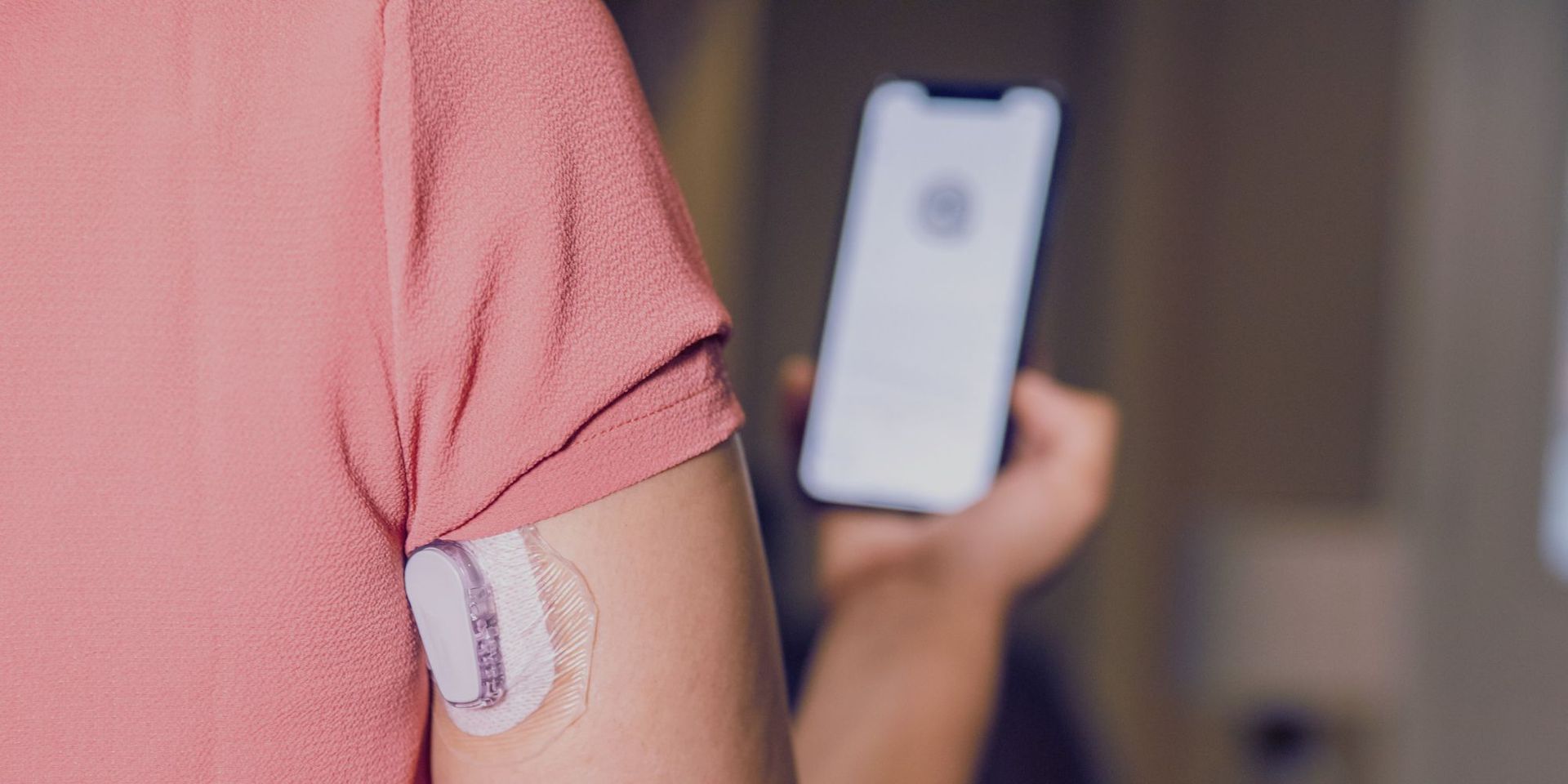
2. Remote Monitoring
Remote patient monitoring is another benefit of connected devices for clinical trial participants. As a result, experts are better equipped to identify any changes in the patient's health and deliver prompt therapies that enhance patient outcomes. Additionally, remote monitoring lessens the need for patients to go to hospitals, which enhances patient satisfaction and lowers healthcare expenses.
3. Wearable Devices
The pharmaceutical industry is also using wearable technology to track patient adherence to medications. These gadgets check medicine usage and alert patients to take their medications on schedule. It results in better patient outcomes and lowers medical expenses.
Potential Challenges & Limitations Of Connected Devices In The Pharmaceutical Industry
1. Data Privacy and Security
In the pharmaceutical sector, connected devices may be used to gather private patient information. It is critical to ensure that all sensitive data is secured against theft and unauthorised access.
2. Regulatory Hurdles
Several laws and regulations apply to connected device use in the pharmaceutical industry. Making sure that these rules are followed is crucial.
3. Patient Acceptance
Not all patients may be willing to use IoT devices for various reasons such as cost or privacy concerns.
4. Technical Issues
Connected devices are susceptible to technical concerns such as device malfunction or connectivity issues. To handle these problems swiftly, it is essential to have solutions in place.
Benefits Of Connected Devices In The Pharmaceutical Industry
1. Improved Patient Outcomes
Healthcare professionals may now keep an eye on patients in real-time thanks to connected IoT devices in the pharmaceutical industry. This implies that medical professionals can monitor patient health indicators including blood pressure, heart rate, and oxygen saturation. A better patient experience can result from using this data to make well-informed decisions about patient care.
2. Increased Efficiency
Healthcare practitioners can concentrate on more important activities by using IoT devices to automate a variety of tasks. Remote patient monitoring, for example, can reduce the need for patients to attend healthcare facilities for routine check-ups, freeing up significant time for healthcare practitioners.
3. Reduced Costs
By removing the need for pointless visits to medical facilities, connected devices can help lower healthcare expenditures. Remote patient monitoring can help identify health problems early so they can be treated before it worsens and become more expensive to treat.
4. Enhanced Data Collection & Analysis
Countless pieces of patient health data can be gathered via connected devices. Better treatment strategies and health issue predictions can be made using this data.
5. Better Drug Development
Data collection on the effectiveness and safety of medications can be done via connected devices. To create better medications and guarantee patient safety, this information can be exploited.
Way Forward
To sum up, connected devices have significantly changed the pharmaceutical sector in a number of ways. The utilisation of IoT devices for real-time patient data collection, promoting medicine adherence, and improving clinical trials are some of the significant subjects we covered in this article. By enabling more precise diagnoses, personalised treatments, and remote monitoring, these gadgets have helped pharmaceutical companies deliver better patient care.
It is impossible to exaggerate the importance of connected devices for the future of healthcare. Most likely, the use of these devices will increase as a result of ongoing technological improvements. Reduced healthcare expenditures, greater patient participation, and better patient outcomes are potential advantages of this growth. Therefore, cooperation among healthcare professionals, pharmaceutical companies, and technology companies is essential to ensuring the ethical and successful use of connected devices.
In light of this, we can say that the pharmaceutical sector is changing due to the use of connected devices, and the potential advantages of this technology are significant. Pharmaceutical businesses may lower costs, optimise their supply chains, and improve patient outcomes by implementing IoT technologies. To improve their operations and deliver the greatest healthcare solutions, pharmaceutical businesses must investigate and deploy such devices, including Adapt Ideations temperature data loggers.
Check out our IoT devices here to know more about our solutions.
Share Our Post.
Awards & Recognition

Best Temperature Monitoring Solution Provider
Awarded by India Biologics & Vaccines Outstanding Industry Awards 2022

Adapt Ideations Recognised As A Supply Chain Leader
by Alcott Global on Supplify's Supply Chain Tech Map 2.0

Related Articles.