Pharmaceutical Supply Chain Security: A Global Imperative
The Global Imperative for Pharmaceutical Supply Chain Security
Analyzing Current Supply Chain Challenges
Risks in Pharmaceutical Supply Chains
Real-Time Visibility in Pharma Supply Chains
Understanding Pharmaceutical Supply Chain Compliance
Regional Variations and Standards
Australia: Code of Good Wholesaling Practice for Medicines
United States: Drug Supply Chain Security Act (DSCSA)
Impact on End-to-End Visibility
Compliance Challenges in the Pharma Industry
Managing Pharma Supply Chain Risks
Identifying Risks in the Pharmaceutical Supply Chain
Building Resilience in Pharma Supply Chains
Strategies for Risk Mitigation
Technology Solutions for Pharmaceutical Supply Chain Security
Role of Technology in Enhancing Security
IoT Devices for Real-Time Monitoring
Blockchain & Its Impact on Pharma Supply Chains
Data Analytics for Risk Prediction & Prevention
Adapting to the Future: Adapt Ideations’ KELVIN and PIXEL Solutions
Introduction to Adapt Ideations' Solutions
Real-Time Monitoring & Visibility with KELVIN
Achieving Compliance Standards with PIXEL
Conclusion: Embracing the Future of Pharmaceutical Supply Chain Security
In the expansive realm of global pharmaceutical supply chains, the significance of security is paramount. The imperative for real-time visibility, essential for maintaining product integrity and compliance across diverse regions, has escalated. The biopharmaceutical and life sciences industry, now
valued at an impressive $1587.05 billion, grapples with challenges in cold chain logistics that contribute to
an annual waste of $37 billion. Within this industry, the consequences of a compromised supply chain are monumental, emphasizing the critical need for stringent security measures. Real-time visibility transcends convenience; it is an absolute necessity, serving as the linchpin to guarantee the safe arrival of high-value, temperature-sensitive medicines and vaccines. Navigating varied regulatory landscapes demands a proactive approach, turning the pursuit of product integrity and compliance into a complex puzzle with distinct requirements in different regions.
The Global Imperative for Pharmaceutical Supply Chain Security
Analyzing Current Supply Chain Challenges
Global Pharma Market Dynamics
The global pharmaceutical market, valued at $1587.05 billion, is shaped by dynamic forces influencing production, distribution, and consumption. Rapid advancements in medical research, evolving healthcare policies, and shifting demographics contribute to the ever-changing landscape. Understanding these dynamic market forces is essential for navigating challenges and ensuring the security and integrity of the pharmaceutical supply chain.
Risks in Pharmaceutical Supply Chains
The pharmaceutical supply chain encounters a variety of risks, necessitating a nuanced approach to fortify its resilience.
- Rapid Temperature Fluctuations: Throughout transit and storage, pharmaceutical products are susceptible to sudden temperature changes, compromising their efficacy and safety.
- Exposure to Light & Heat: High-value medicines and vaccines are vulnerable to damage when exposed to excessive light and heat, emphasizing the need for protective measures.
- Shocks & Packaging Viability: The risk of shocks during transportation can compromise the protective packaging of pharmaceutical products, affecting their overall viability and integrity.
These risks underscore the critical need for comprehensive security measures to safeguard product integrity and ensure the seamless flow of medications through the supply chain.
Real-Time Visibility in Pharma Supply Chains
Real-time visibility is about the instant capture and analysis of supply chain data. It is a game-changing concept for the pharmaceutical industry. This concept is emerging as a transformative solution in addressing the complex challenges inherent in pharmaceutical supply chains.
Numerous pharmaceutical companies have embraced real-time visibility with remarkable outcomes. Temperature Data Loggers empower pharmaceutical companies to deliver at their best, even in challenging environments, making it an indispensable solution for modern supply chain management.
Understanding Pharmaceutical Supply Chain Compliance
Regional Variations and Standards
Pharmaceutical supply chain compliance isn't a one-size-fits-all scenario. Different regions have distinct regulations and standards governing the transportation and storage of temperature-sensitive products.
Australia: Code of Good Wholesaling Practice for Medicines
- Emphasizes criteria for third-party partners during pharmaceutical transport.
- Stresses the importance of monitoring temperature-sensitive medications during transit to meet manufacturer-specified requirements.
United States: Drug Supply Chain Security Act (DSCSA)
- FDA mandate requiring tracking of all medicinal products from 2023 onward.
- Enhances traceability to combat counterfeiting and ensure product safety.
Global Variances
- Diverse standards pose a challenge for companies operating on a global scale.
- Variations in temperature control, documentation requirements, and monitoring protocols are evident across different regions.
Need for Tailored Approaches
- Acknowledges the importance of tailoring compliance approaches based on specific regional regulations.
- Businesses must understand and adhere to the unique standards of each region to ensure seamless pharmaceutical supply chain operations.
Impact on End-to-End Visibility
- Regional variations directly impact end-to-end visibility in the supply chain.
- Lack of standardization hinders comprehensive visibility, making it crucial for companies to adapt and comply with specific regional standards.
Strategic Considerations
- Companies need to strategically align with regional standards to avoid bottlenecks and disruptions in the supply chain.
- Compliance with varied regulations becomes a cornerstone for maintaining product integrity and ensuring the safe delivery of pharmaceuticals globally.
In conclusion, regional variations and standards play a pivotal role in shaping pharmaceutical supply chain compliance. Understanding the intricacies of regulations in specific regions is essential for companies aiming to navigate the global pharmaceutical landscape successfully.
Compliance Challenges in the Pharma Industry
Compliance in the pharmaceutical industry is a multifaceted challenge. This subsection addresses common hurdles faced by pharmaceutical companies, such as the need for real-time monitoring, the complexity of documentation, and the integration of new technologies to meet evolving compliance standards. By addressing these challenges head-on, companies can enhance their supply chain resilience and maintain product integrity.
Read more about - A Deep Dive Into Pharmaceutical Supply Chain Compliance.
Managing Pharma Supply Chain Risks
Identifying Risks in the Pharmaceutical Supply Chain
Supply Chain Vulnerabilities
- Inherent vulnerabilities within the pharmaceutical supply chain encompass transportation, storage, and handling.
- Challenges include exposure to temperature fluctuations, mishandling during transit, and potential deviations from recommended storage conditions.
External Threats & Risks
- External threats pose substantial risks to pharmaceutical products.
- These threats may include theft, tampering, and unforeseen events like natural disasters that can disrupt the supply chain.
Building Resilience in Pharma Supply Chains
Strategies for Risk Mitigation
- Implementing robust risk mitigation strategies is vital.
- Strategies involve leveraging advanced monitoring technologies, employing secure packaging, and conducting comprehensive risk assessments at various stages of the supply chain.
In summary, managing pharmaceutical supply chain risks involves identifying vulnerabilities, addressing external threats, and building resilience through strategic risk mitigation measures. By implementing these strategies and learning from resilient supply chain case studies, companies can enhance the security and reliability of their pharmaceutical supply chains.
Read more about - The Power Of Resilient Supply Chains: A Global Perspective.
Technology Solutions for Pharmaceutical Supply Chain Security
Role of Technology in Enhancing Security
The integration of technology plays a pivotal role in fortifying the security of pharmaceutical supply chains. Leveraging innovative solutions can address inherent challenges and elevate the overall efficiency of operations.
IoT Devices for Real-Time Monitoring
- Leveraging IoT devices, such as Adapt Ideations' KELVIN and PIXEL solutions, plays a pivotal role in providing real-time monitoring for pharmaceutical supply chains.
- These devices enable continuous tracking, offering insights into temperature conditions, ensuring product integrity, and mitigating risks during transit.
Blockchain & Its Impact on Pharma Supply Chains
- Blockchain technology provides an immutable and transparent ledger, enhancing the traceability and security of pharmaceutical products.
- By creating a decentralised and tamper-resistant record of transactions, blockchain minimizes the risk of counterfeiting and unauthorised access.
Data Analytics for Risk Prediction & Prevention
- Data analytics tools analyze vast sets of data to predict and prevent potential risks within the supply chain.
- Predictive analytics can anticipate deviations in temperature, enabling proactive measures to maintain product quality.
Adapting to the Future: Adapt Ideations’ KELVIN and PIXEL Solutions
We at Adapt Ideations introduce our innovative IoT solutions tailored to meet the evolving needs of pharmaceutical supply chains.
Introduction to Adapt Ideations' Solutions
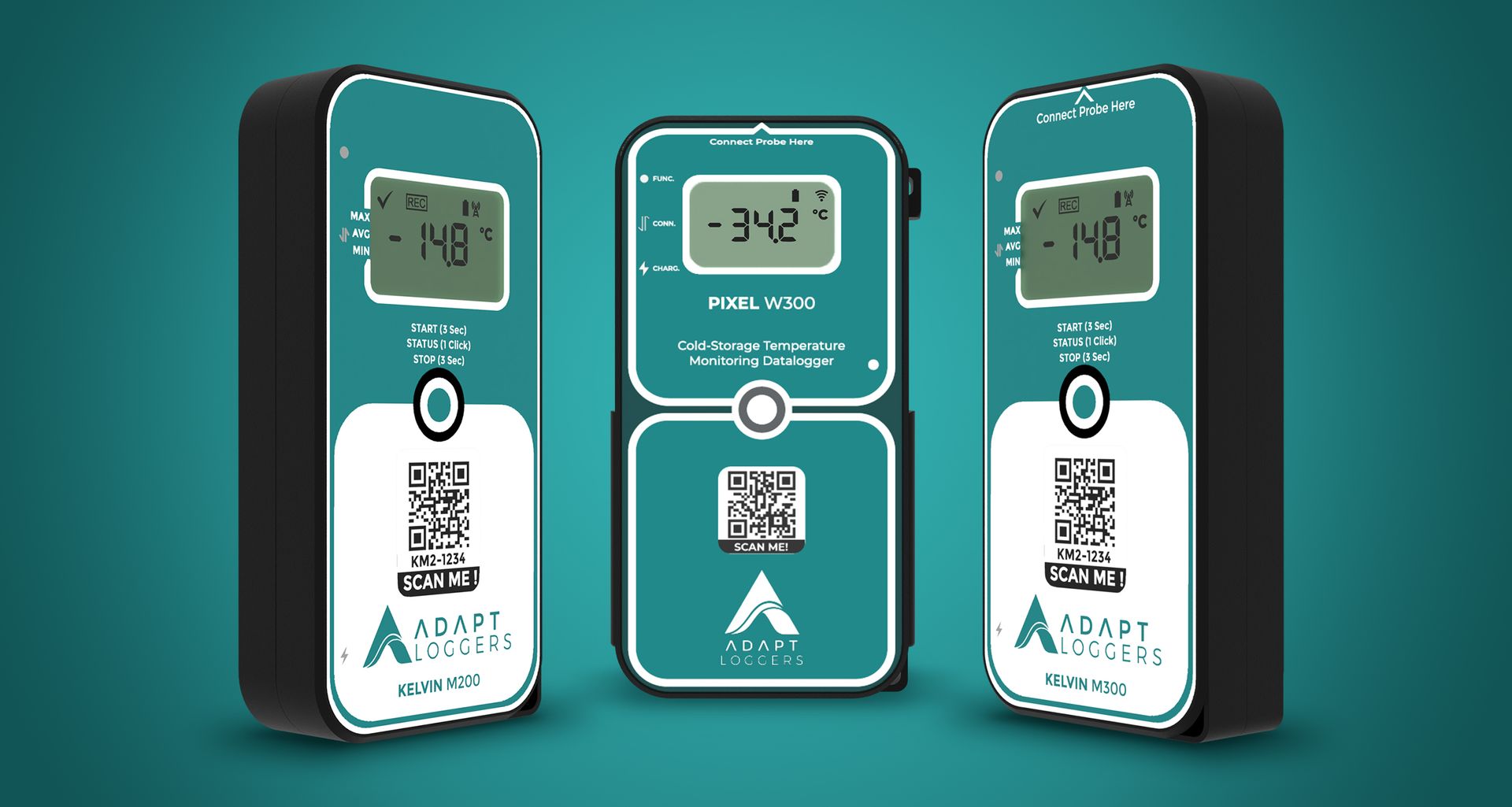
- KELVIN IoT Monitoring Track and Trace Devices and PIXEL Temperature Data Loggers are at the forefront of real-time visibility solutions.
- These solutions cater to the specific requirements of pharmaceutical supply chains, ensuring product integrity and compliance.
Real-Time Monitoring & Visibility with KELVIN
- KELVIN devices provide comprehensive real-time monitoring and visibility at every stage of the pharmaceutical supply chain.
- Features include real-time tracking, instant alerts for temperature deviations, and historical data analysis for informed decision-making.
Achieving Compliance Standards with PIXEL
- PIXEL Temperature Data Loggers are designed to meet the stringent compliance standards of cold storage operations.
- These devices contribute to sustainability efforts while ensuring real-time visibility, compliance, and efficiency in pharmaceutical supply chains.
In essence, technology solutions, such Adapt Ideations' KELVIN and PIXEL solutions, pave the way for a secure and future-ready pharmaceutical supply chain. The seamless integration of these technologies addresses challenges, ensures compliance, and provides real-time visibility, marking a transformative step towards the future of pharmaceutical logistics.
Conclusion: Embracing the Future of Pharmaceutical Supply Chain Security
In conclusion, the future of pharmaceutical supply chain security hinges on embracing real-time visibility, safeguarding product integrity, and adhering to global compliance standards. As challenges persist in this dynamic industry, our solutions, including KELVIN and PIXEL, emerge as key players in ensuring efficiency and compliance. Addressing logistics pain points and offering real-time monitoring, these solutions pave the way for a secure pharmaceutical landscape.
Enquire to find out more about our innovative solutions and how they can be utilized at enquiries@adaptideations.com
Share Our Post.
Awards & Recognition

Best Temperature Monitoring Solution Provider
Awarded by India Biologics & Vaccines Outstanding Industry Awards 2022

Adapt Ideations Recognised As A Supply Chain Leader
by Alcott Global on Supplify's Supply Chain Tech Map 2.0

Related Articles.